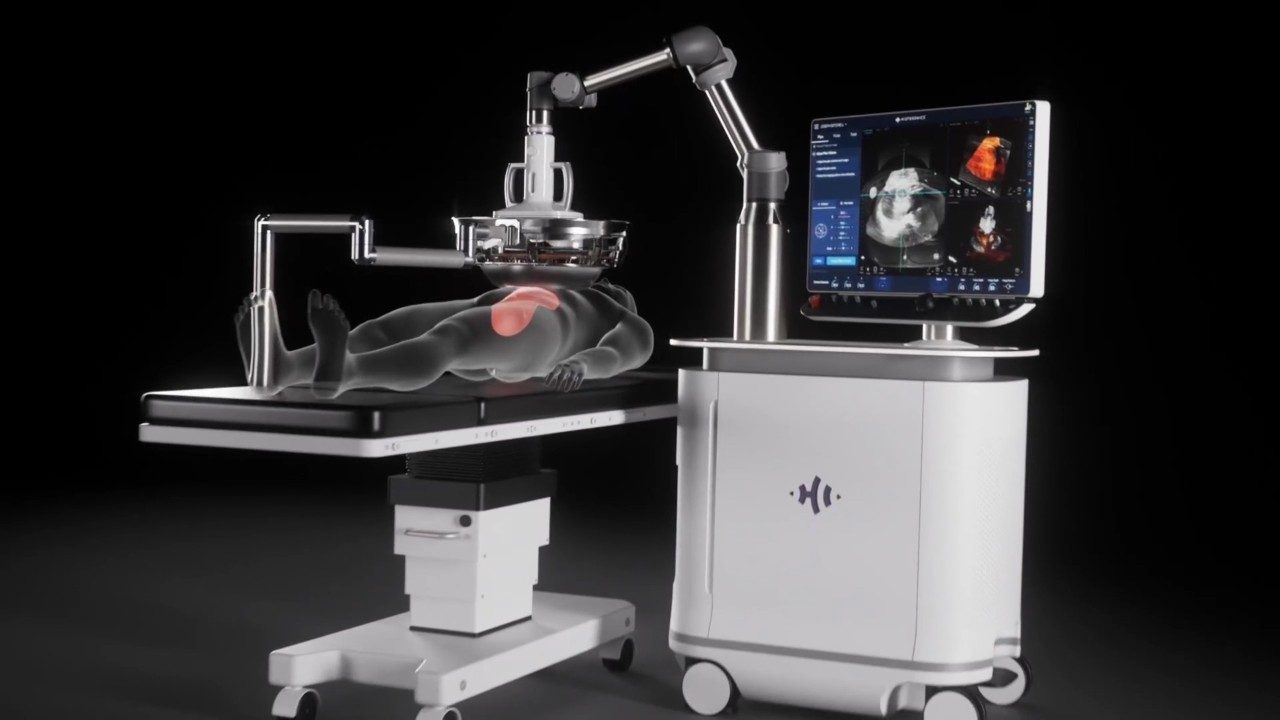
Newswise — In a groundbreaking achievement for cancer treatment, research, and medical technology, the U.S. Food and Drug Administration (FDA) has granted approval for the HistoSonics’ Edison histotripsy device to treat liver tumors. Submitted for marketing authorization through the FDA’s De Novo Classification Request process, an intense market review for medical devices with no existing equivalent, the Edison will be the initial and only histotripsy platform available in the country.
The cutting-edge medical device and its technology were developed in part by Kendall and Laura Hendrick Junior Faculty Fellow Eli Vlaisavljevich, through collaboration with the University of Michigan and HistoSonics, a private medical device company.
Improving treatment for patients and doctors
According to HistoSonics, the Edison uses advanced imaging to deliver personalized, noninvasive histotripsy treatments with precision and control. FDA approval signifies a remarkable step forward in medical innovation and will open access for the technology to be purchased by hospitals and treatment centers for patients battling liver cancer.
The proprietary technology provides several advantages to both patients and the doctors treating them. Treating cancer without radiation or chemotherapy means less pain, shorter recovery times, and reduced risk of complications for patients. The high-intensity focused ultrasound used in histotripsy offers exceptional precision for doctors, allowing them to target diseased tissues with significant accuracy. And because the treatment is noninvasive, patients experience fewer side effects compared to traditional surgical procedures. There is no incision, reducing the risk of infection and scarring.
In addition, preliminary findings indicate that histotripsy can potentially trigger an immune response where other untreated cancer cells are removed by the body’s natural response to histotripsy. This unexpected result has expanded the potential benefits of histotripsy in cancer treatment.
Targeting cancer with focused precision
Histotripsy, a novel therapeutic technique, targets and destroys diseased tissues, including cancerous tumors, without the need for invasive surgery. Instead of traditional surgical methods or radiation, histotripsy employs the power of high-intensity focused ultrasound to fragment and liquefy targeted tissues. This noninvasive approach enables more precise treatment while minimizing the risks associated with invasive surgery, making it a game-changer in the medical field.
Vlaisavljevich, associate professor in the Department of Biomedical Engineering and Mechanics, was an undergraduate student at Michigan Technological University when he was first introduced to histotripsy through a seminar by University of Michigan researcher Zhen Xu. Xu had just successfully completed the first-ever histotripsy treatment. Intrigued by the technology’s potential, Vlaisavljevich entered the university’s graduate program and began developing histotripsy for liver cancer. His research was driven by a close connection to the disease: his mother, Theresa, died of liver cancer when he was 4 years old.
After receiving his doctoral degree in biomedical engineering, Vlaisavljevich began a two-year position at HistoSonics, where he developed the prototype for what would ultimately become the Edison.
In 2017, Vlaisavljevich joined Virginia Tech, continuing his research on histotripsy for the treatment of liver and other cancers in his Therapeutic Ultrasound and Noninvasive Therapies Laboratory. Continued collaboration with HistoSonics produced the first successful clinical trial for the use of histotripsy to treat liver cancer in Barcelona, Spain, in 2018. The trial was named the THERESA Study, in memory of Vlaisavljevich’s mother. Building on that success, HistoSonics launched the much larger #HopeforLiver trial in both the United States and Europe.
“This is the culmination of a very long process of work by many people in both academia and industry to get it to this amazing milestone,” said Vlaisavljevich.
Advancing the future of histotripsy through collaboration
As the Edison and histotripsy enter the health care arena, both serve as a ray of hope for patients and a testament to the boundless potential of innovative minds working in collaboration.
Vlaisavljevich and his team are currently leading large projects to advance histotripsy for the treatment of other cancers, including pancreatic, breast, bone (osteosarcoma), soft tissue sarcoma, brain, and oral, as well as in other applications.
“FDA approval opens up the potential for histotripsy for all of the other types of cancer that we are working on,” said Vlaisavljevich. “For me personally, it means a lot, too, based on my long history working on this, my mom, and more importantly all the many patients who can now start to be treated with histotripsy.”
Strong academic and industry partnerships helped histotripsy achieve this breakthrough moment in what Vlaisavljevich called a win for “team science.” As he and his colleagues continue to develop histotripsy at Virginia Tech, he said, they remain committed to the university’s interdisciplinary and multi-institutional model of collaborative translation science. It has been critical in developing a potentially revolutionary technology like histotripsy and getting it to patients as soon as possible, he said. And the work’s not done yet.
“This is an exciting milestone and hopefully the first of many applications that will be approved for histotripsy in the future,” said Vlaisavljevich. “Now, we are going after all the cancers."