Newswise — Washington, D.C., July 22, 2022 — The cardiac electrophysiology team at MedStar Washington Hospital Center successfully implanted the first dual-chamber leadless pacemaker system in the Baltimore-Washington region this week. Known as the Aveir™ DR Leadless Pacemaker System, this breakthrough technology is being clinically evaluated as part of a worldwide clinical trial sponsored by Abbott, and MedStar Washington Hospital Center was the only hospital in the region selected to participate. The device is not yet commercially available, and its design specifications are subject to change. Patients with slow heart rates who need pacing therapy are candidates for this trial. In May 2022, the MedStar cardiac electrophysiology team was also the first in the region to implant the Aveir™ VR single-chamber leadless pacemaker.
Patients of any age, but most commonly older people, can experience abnormal slowing of the pulse, which may cause fatigue, dizziness, passing out, and diminished quality of life. Traditional pacing therapy has required threading wires through a vein to the heart and connecting them to a metal, disc-shaped pacemaker implanted under the skin of the chest.
Thanks to the design of this investigational device, treatment can now be delivered by two mini pacemakers—each smaller than a AAA battery—that are inserted via a catheter in the large vein in the thigh. Under X-ray guidance and without any incisions, the two devices are secured within the upper and lower chambers of the heart, respectively, to normalize rhythm. The investigational device has also been designed to be readily retrieved and removed if needed.
“This major leap forward offers patients an excellent option to treat abnormally slow heart rates,” said Zayd Eldadah, MD, PhD, director of Cardiac Electrophysiology at MedStar Health and principal investigator of the Aveir DR i2i clinical trial. “No wires implanted in veins, no metal device under the skin, and not even a surgical incision means the design offers the potential for less risk, greater comfort, and fewer post-procedure restrictions.”
Compared to traditional pacing, leadless technology can eliminate inflammation, scars, and long-term problems, such as wire insulation breaks, vein blockage, and device infection. The procedure typically takes less than an hour, and patients can go home the same day.
“The pacemaker’s impressive battery life is a key benefit,” said Cyrus Hadadi, MD, associate director of Cardiac Arrhythmia Research at MedStar Washington Hospital Center. “It also offers a real-time mapping design capability that allows physicians to measure heart electric signals, offering options to allow for improved positioning of the devices.”
Most patients needing pacemaker therapy require dual-chamber systems to best regulate their rhythm. “In place of wires, the Aveir uses the patient’s own blood to transmit electrical information between the heart’s upper and lower chambers—a very clever and energy-efficient innovation,” added Dr. Eldadah, who performed the region’s first implant on July 19. “This device has the potential to be a transformational advance for patients—perhaps the medical equivalent of graduating from a rotary-dial landline to a smartphone.”
The Aveir ™ DR i2i ™ Study is designed to assess the safety and effectiveness of the Abbott Aveir™ DR Leadless Pacemaker System in patients with abnormal heart rhythms. Patients enrolled in the study will receive Abbott’s Aveir DR leadless pacemaker device through a minimally invasive, catheter-based procedure. It will enroll up to 550 patients at up to 80 sites worldwide and data collected will be submitted to support global regulatory approvals.
###
MedStar Washington Hospital Center is a not-for-profit, 912-bed, teaching and research hospital in the nation’s capital, and is a major referral center for treating the region’s most complex cases. Its cardiology program is highly acclaimed, and its cardiac surgery program has consistently earned the highest national rating–three stars–from the Society of Thoracic Surgeons. MedStar Washington operates the region’s first Comprehensive Stroke Center and the District’s only Cardiac Ventricular Assist Device program, both certified by The Joint Commission. The hospital is also home to MedSTAR, a nationally verified level I trauma center with a state-of-the-art fleet of helicopters and ambulances, and operates the region’s only adult Burn Center.
MEDIA CONTACT
Register for reporter access to contact detailsArticle Multimedia
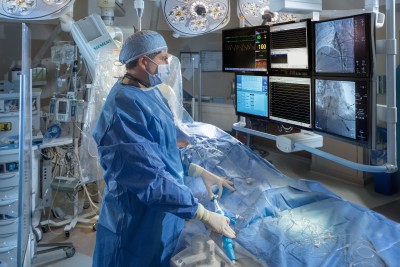
Credit: MedStar Washington Hospital Center
Caption: Dr. Zayd Eldadah in the Cardiac Electrophysiology Lab, implanting the Aveir dual-chamber pacemaker system

Credit: MedStar Washington Hospital Center
Caption: Aveir dual-chamber pacemaker
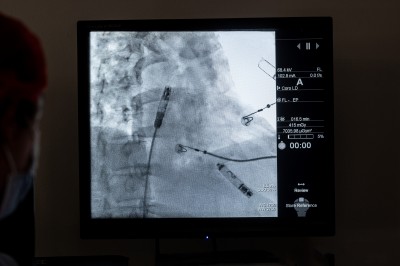
Credit: MedStar Washington Hospital Center
Caption: Dual-chamber pacemakers secured within the upper and lower chambers of the heart



