FINDINGS
Newswise — This study, led by investigators in the Smidt Heart Institute at Cedars-Sinai, applies novel artificial intelligence (AI) methods to measure heart function. Investigators have designed a program that can be applied to standard heart ultrasounds to measure global, longitudinal myocardial strain, which is a marker of muscle function.
The project addresses current problems in measuring myocardial strain, such as large variations in standard measurements and proprietary software, which may require specialized pictures and processing. The program created by Smidt Heart Institute investigators is openly available and can be applied to standard images, making it well suited for large datasets of heart ultrasounds.
EXPERT COMMENTARY
“We are developing deep-learning approaches to extract information from medical images that may not be readily visible to the human eye,” said Alan Kwan, MD, an assistant professor in the Department of Cardiology in the Smidt Heart Institute at Cedars-Sinai and first author of the study. “We want to ensure we are using safe, rigorous, and understandable methods, in order to fully leverage the information from medical imaging and improve clinical care and research.”
BACKGROUND
The function of the heart is frequently measured by ejection fraction, but many physicians say a more precise measurement is cardiac strain—where the actual shortening of the heart muscle is detected to identify subtle findings of cardiac dysfunction. In echocardiography, “speckle tracking” is used to calculate longitudinal strain; however, the application of this method can vary significantly between both machine vendors and sonographers. This variation can impact the reproducibility of strain measurements across multiple studies.
In this study, Smidt Heart Institute investigators built upon prior research where they developed deep-learning algorithms to identify the contour of the left ventricle for measurement of ejection fraction—a calculation of the heart’s ability to pump oxygen-rich blood out to the rest of the body. The investigators then repurposed the tool to measure strain.
The result is an automated, open-source, vendor-agnostic method to retrospectively measure global longitudinal strain to improve research applications and streamline patient analysis.
IMPACT
This deep learning-based strain program is open source and freely available, which makes it broadly applicable to already existing datasets. By providing access to anyone who wishes to use it, Cedars-Sinai researchers aim to address current barriers to access and difficulties with comparison between different brands of strain.
Additionally, because the algorithm is applicable to standard clinical images and does not require specific image acquisition, it is ideal for research application to large clinical databases and can be easily run on existing data.
This study advances the application of deep learning in echocardiography, along with other projects by the investigators that are aimed at improving cardiac patient care, by more accurately identifying hidden signs of cardiovascular disease.
JOURNAL
The research was published in the peer-reviewed journal JACC: Cardiovascular Imaging.
AUTHORS
Cedars-Sinai authors involved in the study are Alan C. Kwan, MD; Ishan Jain; John Theurer, BS; Robert Siegel, MD; Susan Cheng, MD, MMSc, MPH; and David Ouyang, MD. Additional authors are Ernest Chang, MD, PhD; Xiu Tang; Nadia Francisco, MSc; Francois Haddad, MD; David Liang, MD, PhD; Alexandra Fábián, MD, PhD; Andrea Ferencz; Neal Yuan, MD; Béla Merkely, MD, PhD, DSc; Attila Kovács, MD, PhD; and Márton Tokodi, MD, PhD.
FUNDING
Alan C. Kwan reports funding support from the Doris Duke Charitable Foundation Grant 2020059, American Heart Association Career Development Award 23CDA1053659 and NIH R01HL131532-06. Ernest Chang reports funding from National Institutes of Health T32HL097129-13. This study was partially supported by the National Research, Development, and Innovation Fund of Hungary within the framework of the Artificial Intelligence National Laboratory Program and by the Thematic Excellence Program (2020-4.1.1. – TKP2020) of the Ministry for Innovation and Technology in Hungary, within the framework of the Bioimaging thematic program of the Semmelweis University. This work was supported in part by the National Institutes of Health NIH K99 HL157421-01.
Follow Cedars-Sinai Academic Medicine on X, formerly Twitter, for more on the latest basic science and clinical research from Cedars-Sinai.
MEDIA CONTACT
Register for reporter access to contact detailsArticle Multimedia

Credit: Image by Getty.
Caption: Investigators in the Smidt Heart Institute at Cedars-Sinai have developed a new AI tool to standardize cardiac strain measurements.
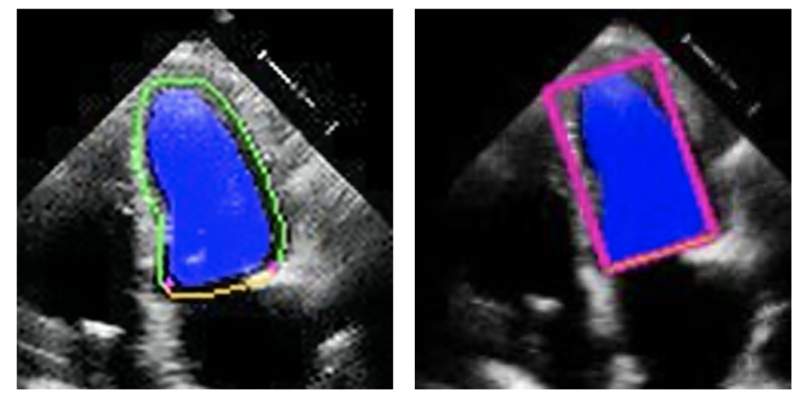
Credit: Image courtesy of Alan Kwan, MD.
Caption: Echocardiogram image demonstrating myocardial strain measurement.

Credit: Cedars-Sinai
Caption: Alan Kwan, MD
